Views: 0 Author: Site Editor Publish Time: 2025-08-15 Origin: Site
Chromium oxide (Cr₂O₃) is a strong refractory ceramic. It performs well in environments with high heat. Many industries use chromium oxide in bricks and other ceramic materials. It is commonly chosen for kilns and furnaces because it does not break down from heat or rust. Chromium oxide is essential in refractory ceramic products that need to last long and function effectively.
Chromium oxide allows factories and manufacturers to handle tough conditions safely and efficiently.
Chromium oxide is a hard ceramic. It can handle high heat and chemicals. This makes it good for bricks in kilns and furnaces. Its special crystal shape keeps it strong and steady. This helps bricks last longer. The bricks can handle fast temperature changes. They do not crack easily. Hydrothermal hydrogen reduction is a cleaner way to make chromium oxide. It makes less pollution than old methods. Chromium oxide makes ceramic bricks stronger. It helps them last longer in tough places. This helps factories stay safe and work well. But making and using chromium oxide needs care. Toxic hexavalent chromium must be controlled. This keeps people and nature safe.
Chromium oxide is very important in refractory ceramics. Factories use it because it can handle high heat and strong chemicals. You will find chromium oxide in ceramic refractory bricks and other materials. It helps keep factories safe and working well. Its special chemical structure and new ways of making it make it a top pick for strong refractory ceramics.
Chromium oxide has the chemical formula Cr₂O₃. It is part of the refractory ceramic materials group. This compound has a trigonal crystal structure. That makes it very stable and strong. The way chromium and oxygen atoms are arranged creates a thick, tough lattice. This structure does not bend or break easily, even with lots of heat and pressure.
| Compound | Chemical Formula | Crystal Structure | Crystal System | Space Group |
|---|---|---|---|---|
| Chromium(III) oxide | Cr₂O₃ | Trigonal | Trigonal | R3c |
The crystal system helps chromium oxide work well in ceramic refractory bricks. The trigonal structure lets it keep its shape inside furnaces and kilns. This is important for refractory ceramics that get hot and cool many times.
The electronic structure of chromium oxide also matters. Scientists found that chromium oxide films grow in layers. They form an O–Cr–O trilayer with a Cr₂O₃ honeycomb plane on top. This setup changes the density of states near the Fermi level. It affects the workfunction, stability, and magnetic features. Charge-transfer between oxide layers and metal bases helps keep the film stable and working well in refractory ceramic materials.
Tip: The special crystal and electronic structure of chromium oxide make it great for refractory ceramics. It works best where strength and heat resistance are needed.
Chromium oxide’s physical and chemical properties make it good for industry. It can handle high heat, is very hard, and resists chemicals. These traits make it perfect for ceramic refractory bricks and other materials used in steelmaking, glass, and chemical plants.
| Property / Characteristic | Description / Value | Influence on Industrial Use |
|---|---|---|
| Heat Stability | High heat stability | Suitable for ceramic refractory bricks and materials exposed to high temperatures |
| Hardness | Hard mineral (chromite hardness ~4.5 Mohs) | Used in hard rustless steel and durable coatings |
| Chemical Composition | FeCr₂O₄ with ~68% Cr₂O₃ content | Primary source of chromium for metallurgical and chemical uses |
| Oxidation States | Multiple oxidation states, including hexavalent (toxic) and trivalent (less toxic) | Oxidation states affect catalytic activity and toxicity, influencing catalyst design and handling safety |
| Catalytic Activity | Active metal sites for polymerization catalysts | Used in polymerization catalysts for producing HDPE and LLDPE |
| Crystal Structure | Isometric, no cleavage, brittle tenacity | Physical robustness supports use in refractory and metallurgical applications |
| Specific Gravity | 4.5 to 4.8 | Affects processing and separation techniques in ore refining |
| Toxicity | Hexavalent chromium compounds are toxic | Safety considerations in handling and application selection |
Chromium oxide’s trigonal crystal system and electronic structure help ceramic refractory bricks last longer. They can fight off wear, rust, and heat shock. These features are needed for refractory ceramics in tough jobs.
There are different ways to make chromium oxide for refractory ceramic materials. The method chosen changes how much is made, how fast, and how it affects the environment. The main ways are traditional roasting, hydrothermal hydrogen reduction, and other reduction methods.
| Production Method | Description | Yield / Efficiency | Environmental Impact / Notes |
|---|---|---|---|
| Traditional Method | High-temperature oxidation roasting of chromite ore with sodium carbonate to produce chromium salts (e.g., sodium dichromate), followed by leaching, acidification, crystallization; Cr₂O₃ prepared by mixed roasting of sodium dichromate with ammonium sulfate or sulfur, or thermal decomposition of chromic anhydride (CrO₃). | Exact numerical yields not explicitly stated. | Generates Cr(VI)-containing pollutants (Na₂SO₄, NaHSO₄) that are discharged and not recycled, causing environmental pollution. |
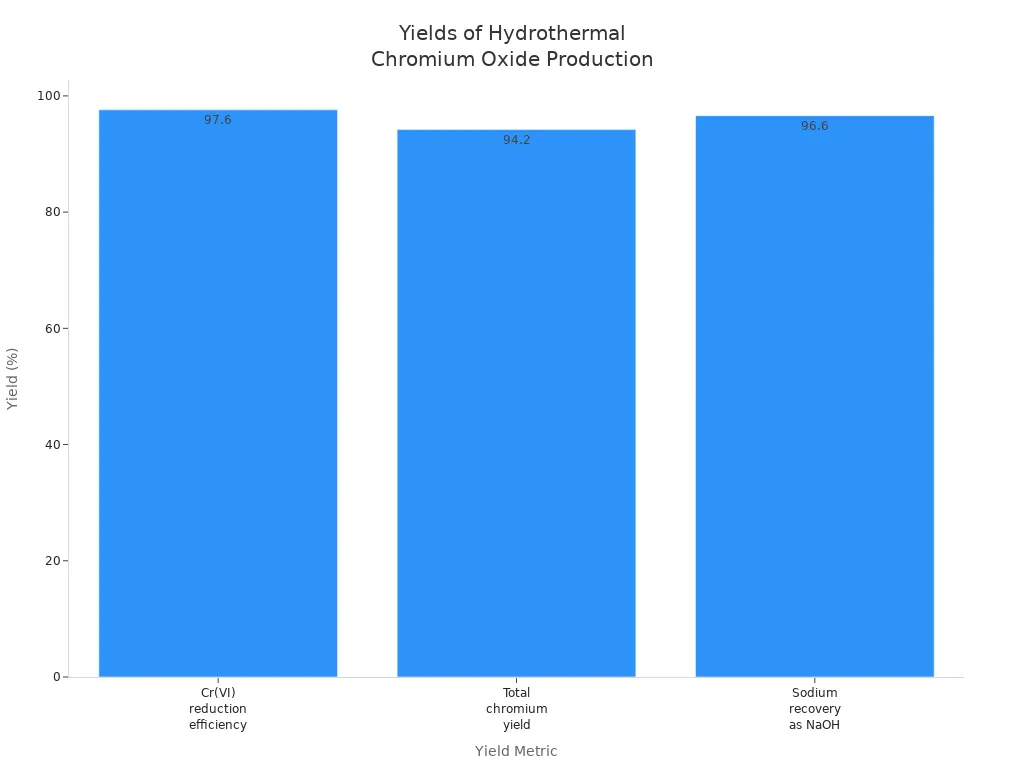
| Hydrothermal Reduction of Sodium Chromate | Hydrothermal reduction of Na₂CrO₄ aqueous solution with hydrogen, followed by thermal decomposition of chromium oxide hydroxide (CrOOH) to Cr₂O₃. | Cr(VI) reduction efficiency: 97.6% | |
| Total chromium yield from Na₂CrO₄ to Cr₂O₃: ~94.2% | |||
| Sodium recovery as NaOH: 96.6% | Cleaner process with recycling of sodium as NaOH, reducing Cr(VI) pollution and enabling a shorter, integrated production route. | ||
| Other Methods | Carbon reduction, ethanol reduction, ammonium salt reduction, hydrogen reduction of chromate salts, microwave plasma treatment, solution combustion, mechanochemical processing, sol-gel, electro-reduction, etc. | Generally lower efficiency and higher cost; specific yields not detailed. | Various advanced techniques with potential but less industrially established or efficient compared to hydrothermal hydrogen reduction. |
The traditional way uses high heat to roast chromite ore with sodium carbonate. This makes chromium salts. These salts are changed into chromium oxide by leaching, acidification, and crystallization. This method has been used for many years. But it makes a lot of waste and pollution, especially Cr(VI) compounds. These are bad for people and the environment.
Hydrothermal hydrogen reduction is a cleaner way. It uses hydrogen gas to reduce sodium chromate or potassium chromate. This gives high amounts of chromium oxide. The process also recycles sodium hydroxide, so there is less waste. Hydrothermal reduction gets a Cr(VI) reduction efficiency of 97.6%, a total chromium yield of about 94.2%, and sodium recovery of 96.6%. These numbers make it a better choice for making refractory ceramic materials.
Other reduction methods, like carbon or ethanol reduction, are used too. But they usually make less product and cost more. New ways like microwave plasma treatment and sol-gel processing look promising. They are not used much for making ceramic refractory bricks yet.
How much pollution is made matters when picking a production method for chromium oxide. Recovery methods, like recycling chromium from leather tanning leftovers, help the environment more than making chromium from scratch. These ways cut climate change, human harm, and damage to nature by over 90%.
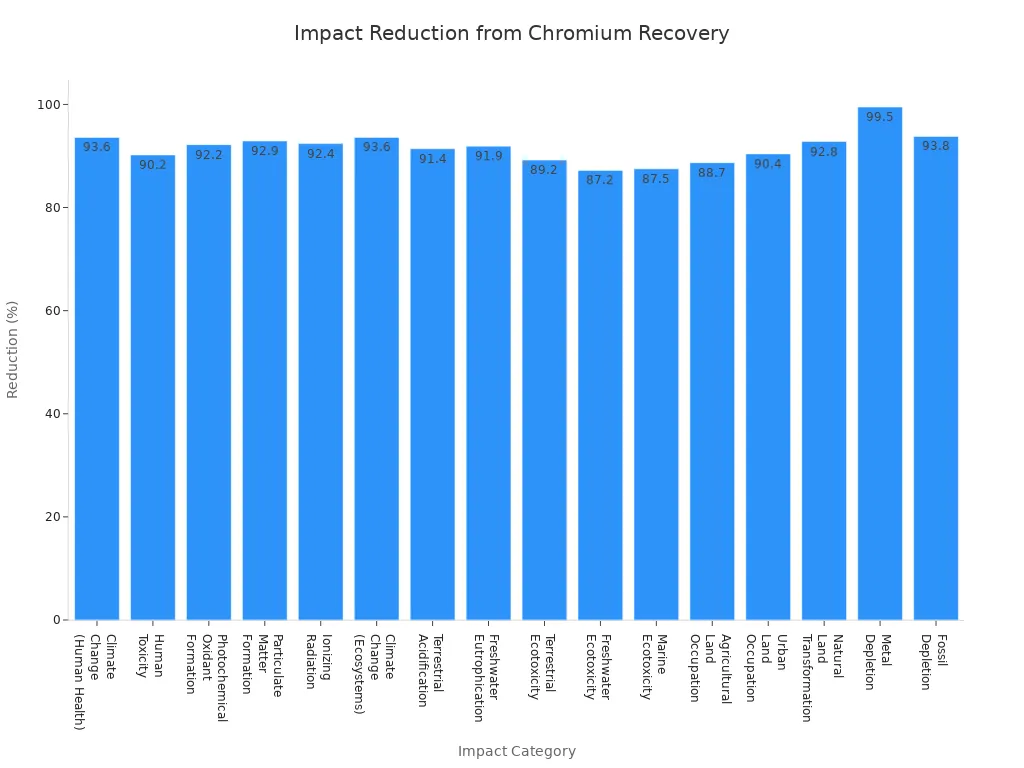
Note: Hydrothermal hydrogen reduction makes more chromium oxide and is better for the environment. It is a good choice for making refractory ceramics and ceramic refractory bricks.
Companies like Yufeng Refractory use new ways to make high-quality chromium oxide for ceramic refractory bricks and other materials. These products are used in steelmaking, glass, and chemical plants. Refractory ceramics must work well in very hot places.
Chromium oxide has been made for a long time. Since the late 1800s, industries have used it for high heat jobs. New methods keep making it better and safer. Today, ceramic refractory bricks with chromium oxide help factories stay safe and work well, even in tough conditions.
Chromium oxide is very important in refractory ceramics. It helps ceramic bricks work well in places with high heat. Its special features make it needed in many industries. The next parts show why chromium oxide does so well in tough jobs.
Chromium oxide can handle very high heat. It melts at about 1990°C. In tests, even with borax frit, it does not melt in a crucible. This shows it is better at resisting heat than copper or cobalt oxides. Those melt at much lower temperatures.
The crystal structure of chromium oxide helps it stay strong. The trigonal lattice stops it from expanding too much. This keeps it from cracking or breaking. Even above 2000°C, it keeps its shape and strength. That is why it is used in bricks for kilns and furnaces.
Chromium oxide’s high melting point and strong structure help bricks last through many heating and cooling cycles.
Scientists found that adding yttrium makes chromium oxide even stronger. Yttrium builds a thick, layered oxide. This cuts down empty spaces and controls how much the material grows. It helps the ceramic resist damage from quick temperature changes. Yttrium also makes grain boundaries stronger and helps oxide films stick better. This makes the ceramic last longer.
The crystal structure of chromium oxide stops expansion from heat.
It makes a thick oxide layer at high heat, which protects it.
This layer keeps the material safe from chemicals.
These features show why factories use chromium oxide in bricks for glass furnaces and reactors. It stays solid and safe at high heat, helping factories run well.
Chromium oxide is also good at fighting chemicals. This helps bricks stand up to strong chemicals in factories.
Chromium oxide makes a tough film in acids like sulfuric acid. This film keeps the material from rusting until Cr6+ ions form. In salty solutions, chromium oxide stays in the film. It works with other oxides to stop corrosion. Chromium oxide stays stable in many chemical conditions. It does not break down easily.
| Chemical Environment / Chemical Species | Chromium Oxide Resistance Evidence | Quantitative Measure / Indicator |
|---|---|---|
| Acidic environments (e.g., sulfuric acid) | Makes a strong film, stops rust until Cr6+ forms | Formation energy: −6.5 eV per O2 molecule |
| Chloride-containing solutions (e.g., NaCl) | Stays in the film, helps stop corrosion | Stable Cr2O3 presence; pH stays steady |
| Redox and pH conditions | Stays strong in many conditions; breaks down when Cr6+ forms | pH drops a lot when it dissolves |
| Alloying context (mixed oxides) | Works with other oxides to fight corrosion | Formation energies: Al2O3 (−10 eV), Cr2O3 (−6.5 eV) |
Chromium oxide does not dissolve much in slag or melted metals. This means bricks with chromium oxide last longer and need less fixing. When it mixes with alumina, it stops alumina from breaking down in slag. This makes it even better at fighting corrosion.
Chromium oxide fights acids, salts, and strong chemicals.
It stays strong in both acid and base conditions.
Its film keeps chemicals from hurting the material.
These traits make chromium oxide a top pick for bricks in steel, glass, and chemical plants.
Bricks need to be strong to work in tough places. Chromium oxide makes ceramic bricks tougher. It helps them hold up under heavy loads and shocks.
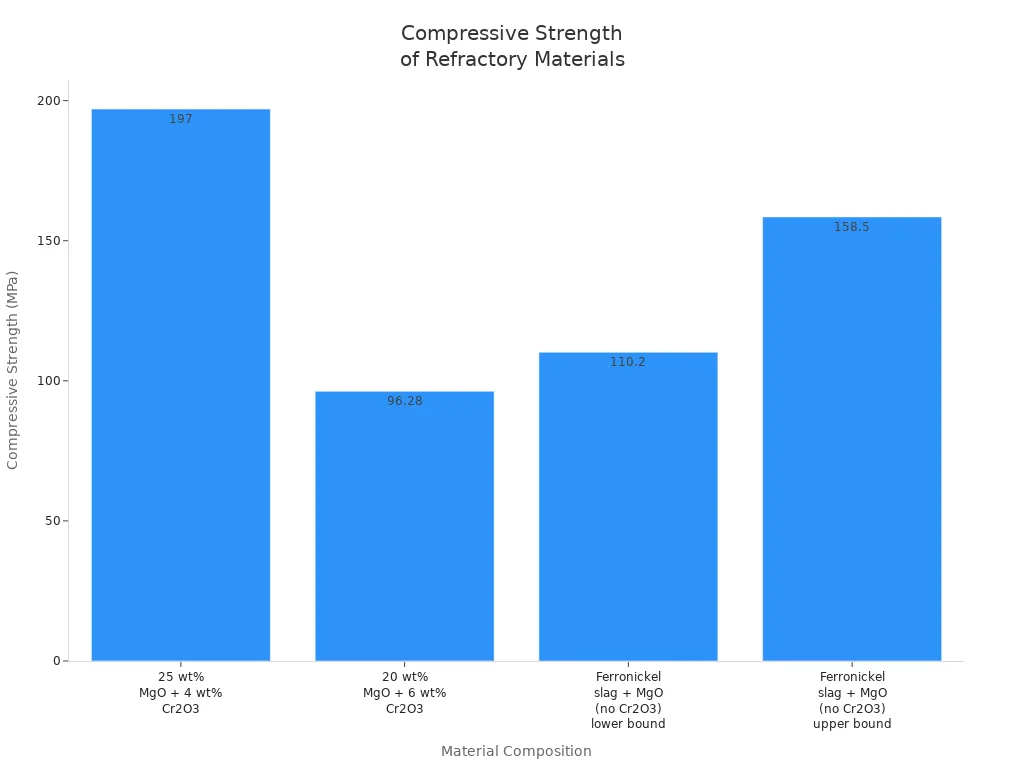
Tests show that adding chromium oxide to magnesia bricks makes them stronger. For example, mixing 25% magnesia and 4% chromium oxide and heating it with microwaves gives a strength of 197 MPa. This is much higher than bricks without chromium oxide. Even with regular heating, chromium oxide makes bricks stronger and more heat-resistant.
| Material Composition | Sintering Method | Temperature (°C) | Time | Compressive Strength (MPa) | Additional Properties |
|---|---|---|---|---|---|
| 25 wt% sintered magnesia + 4 wt% Cr2O3 | Microwave sintering | 1350 | 20 minutes | 197 | Twice the shock resistance; bulk density 2.97 g/cm³; porosity 1.4% |
| 20 wt% sintered magnesia + 6 wt% Cr2O3 | Conventional sintering | 1350 | 3 hours | 96.28 | Heat resistance 1840°C; bulk density 2.68 g/cm³; porosity 15.19% |
| Ferronickel slag + magnesia (no Cr2O3) | Conventional sintering | 1350 | 3 hours | 110.2 - 158.5 | Lower heat resistance and strength than bricks with chromium oxide |
Chromium oxide does more than make bricks strong. It also helps them handle quick temperature changes and lowers porosity. This means bricks do not crack or break easily.
Chromium oxide makes grain boundaries stronger in ceramics.
It lowers porosity, so the material is tougher.
Bricks last longer and work better.
These strengths show why factories trust chromium oxide for bricks in places like steel furnaces.
When you put together heat resistance, chemical strength, and toughness, chromium oxide becomes a key part of modern refractory ceramics.
Chromium oxide is very important in metallurgy. Factories use it to make steel and alloys stronger. It helps metals resist rust and stay tough. Chromium oxide makes a shield on stainless steel. This shield stops corrosion and keeps the metal strong, even at high heat. It works up to 800°C. Steel plants use chromium oxide in bricks and molds. These bricks can take heat above 2000°C. Chromium oxide is also used for machines, tanks, and armored vehicles. Scientists are testing it for nuclear and hydrogen power systems. It does not break down from heat or rust. Some companies try mixing chromium oxide with other materials. They want to use it in turbines and containers for melted metal.
Chromium oxide helps metals last longer and stay strong in tough places.
Ceramics makers use chromium oxide to make bricks better. It helps bricks reflect heat and fight chemicals. Chromium oxide is added to bricks to make them last longer. The size of chromium oxide particles matters. Small particles spread well but may lower heat resistance. The firing temperature also changes how it works. Chromium oxide mixes well with aluminum and magnesium oxides. This makes it important for bricks in kilns, furnaces, and reactors.
Chromium oxide helps ceramic products last longer.
It lets bricks survive many heating and cooling cycles.
Chromium oxide is a common pigment in paints and coatings. In 2024, it was about 36% of the market for coatings and paints. It gives a bright green color and is strong against chemicals. People use it for building, industry, and car paints. The pigment type of chromium oxide can take heat and does not wear away easily. As more cars are made, more chromium oxide pigment is needed. Companies use it to make coatings that look good and last long.
| Application Area | Benefit of Chromium Oxide Pigment |
|---|---|
| Architectural Paints | Bright color, stands up to weather |
| Industrial Coatings | Fights chemicals and scratching |
| Automotive Finishes | Handles heat, keeps color for a long time |
Abrasive products use chromium oxide because it is very hard. Makers put it in polishing compounds, grinding wheels, and sandpaper. Chromium oxide abrasives polish metal, glass, and gems. Its toughness helps remove scratches and make smooth surfaces. Metal workers and jewelry makers use chromium oxide for fine finishing.
Chromium oxide abrasives help make hard materials smooth and shiny.
Chromium oxide gives many good things to refractory ceramics. It is special because it can take a lot of heat. Many factories pick chromium oxide for their bricks. These bricks do not melt or break at high temperatures. This makes them great for kilns, furnaces, and reactors.
Chromium oxide bricks work well in very hot places.
They do not crack when the temperature changes fast.
These bricks last longer because they do not wear down easily.
Chromium oxide keeps bricks safe from rust and scratching.
Chromium oxide reacts with slag in furnaces. It makes a strong spinel layer on the bricks. This layer stops slag from getting inside. The bricks last longer and need fewer repairs. Bricks with alumina and chromium oxide work better than others. They are best for copper smelting and waste melting. When used right, chromium oxide can make bricks last twice as long.
| Aspect | Benefit |
|---|---|
| High Temperature Resistance | Handles heat above 1990°C |
| Thermal Shock Resistance | Stays strong when heated and cooled fast |
| Corrosion Resistance | Makes layers that block slag and chemicals |
| Longevity | Helps bricks last longer |
Chromium oxide helps factories stay safe and work better. It makes bricks stronger and more dependable.
Chromium oxide also has some problems. One big problem is it can hurt people and nature. Hexavalent chromium, a type made during use or waste, can pollute soil, water, and air. People near this pollution can get sick. They may have stomach pain, trouble breathing, skin rashes, or even cancer. Some places, like Dallas, Texas, have had health problems from chromium.
Hexavalent chromium is bad for people and the earth.
Kids and poor families are often hurt the most.
Slag chemicals can also break the spinel layer. Things like CaO and Na2O in slag can damage the layer. This makes the bricks not work as well. Gases from waste can also hurt the protective parts. This makes bricks not last as long.
Rules now say companies must lower chromium pollution. Groups like the EPA and REACH want cleaner ways to make bricks. Companies must buy new machines to follow these rules. This can cost more money.
New research tries to make purer chromium oxide. Closed-loop recycling helps cut down on waste. Nanotechnology and robots help make better and safer bricks. But it is still hard to balance cost, safety, and how well the bricks work.
Companies must keep making better ways to protect workers and nature. They need to make strong, safe bricks for factories.
Chromium oxide is an important refractory ceramic. It can handle very high heat. It does not get damaged by chemicals. It stays strong for a long time. Many factories use it in bricks, paints, and coatings.
Chromium oxide helps make better metals, ceramics, and coatings.
Its strong features are useful in energy, cars, and buildings.
Trivalent chromium compounds are safer and help follow rules about pollution.
Learning about chromium oxide helps scientists make safer and stronger ceramics for tomorrow.
Chromium oxide helps refractory ceramics handle high heat and strong chemicals. These features let bricks last longer in kilns and furnaces. Many industries pick chromium oxide for reliable ceramic materials.
Factories use these bricks in steelmaking, glass, and chemical plants. The bricks protect equipment from very high heat and harsh chemicals. Yufeng Refractory makes these bricks for many factory jobs.
Chromium oxide makes ceramic materials stronger and tougher. It helps bricks stop cracking and rusting. This keeps ceramics safer and saves money in hot places.
Trivalent chromium oxide is safer than hexavalent chromium. Companies like Yufeng Refractory follow strict safety rules. Good handling and new ways of making bricks lower risks for people and nature.
Many companies recycle bricks with chromium oxide. Recycling cuts down on waste and helps the environment. Yufeng Refractory uses new recycling methods to make greener ceramic materials.